What is Acid rain?
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it has elevated levels of hydrogen ions (low pH). It can have harmful effects on plants, aquatic animals and infrastructure. Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide, which react with the water molecules in the atmosphere to produce acids. Some governments have made efforts since the 1970s to reduce the release of sulfur dioxide and nitrogen oxide into the atmosphere with positive results. Nitrogen oxides can also be produced naturally by lightning strikes, and sulfur dioxide is produced by volcanic eruptions. Acid rain has been shown to have adverse impacts on forests, freshwaters and soils, killing insect and aquatic life-forms, causing paint to peel, corrosion of steel structures such as bridges, and weathering of stone buildings and statues as well as having impacts on human health.
Chemical Processes
Combustion of fuels produces sulfur dioxide and nitric oxides. They are converted into sulfuric acid and nitric acid.[41]
Gas phase chemistry
In the gas phase sulfur dioxide is oxidized by reaction with the hydroxyl radical via an intermolecular reaction:[5]
- SO2 + OH· → HOSO2·
Which is followed by:
HOSO2· + O2 → HO2· + SO3
In the presence of water, sulfur trioxide (SO3) is converted rapidly to sulfuric acid:
- SO3 (g) + H2O (l) → H2SO4 (aq)
Nitrogen dioxide reacts with OH to form nitric acid:
- NO2 + OH· → HNO3
- Limestone and Marble
- CaCO3(s) + H2SO4(aq) -> CO2(g) + H2O(l),, + Ca(NO3)2(aq)
- Metal Corrosion
- Metal + Acid -> Salt + Water
- i.e. Fe(II)(s) + H2SO4(aq) -> FeSO4(aq) + 2H+
Effects of Acid Rain
Acid rain damages plant leaves and has cause mass defoliation of pine forests in Europe and USA. Acid rain changes acidity of lakes and rivers killing fish eggs and pH sensitive plants and organisms.
Soil pH is also affected which leads to the biological magnification of heavy and toxic metals such as aluminium as well as the leeching of minerals vital to the survival of flora. Acid rain also causes damage to concrete, marble, limestone and sandstone buildings and statues (Notre Dame).
Soil pH is also affected which leads to the biological magnification of heavy and toxic metals such as aluminium as well as the leeching of minerals vital to the survival of flora. Acid rain also causes damage to concrete, marble, limestone and sandstone buildings and statues (Notre Dame).
Chemical Equations
It is clear that acid rain has a wide range of negative effects on the natural and built environment. It is only through the regulation of the release of acidic oxides such as SO2 and NO2 that acid rain can be minimised.
How to prevent Acid Rain pollution
The planet that we inherited from our parents is not the same planet that we'll be giving to our children. Through the excessive burning of our nonrenewable fossil fuels in power plants, factories and cars, we have created acid rainpollution, i.e. today's precipitation has high levels of nitric and sulfuric acids. This has devastating effects on our oceans, lakes and rivers and all the animals that inhabit them [source: National Geographic]. Read the tips listed below and learn about the small steps that you can take to prevent acid rain pollution.
- Energy conservation The biggest step that you can take to prevent acid rain is to decrease your energy consumption. Close the lights when you leave the room and turn off computers and televisions when you're not using them. Whenever you're not using an electrical appliance, simply shut it off to conserve energy. Another large consumer of energy is your home's heating and cooling system. Make sure to use your air conditioning only when you really need it. Also, when you leave the house, turn down your heater's thermostat. It will cost you less and save more energy [source: EPA].
- Transportation Because cars are a major contributor to acid rain pollution, it's important to find alternate modes of transportation in an effort to decrease our reliance on fossil fuels. By using public transit, carpools, bikes and even your feet, you're helping reduce auto emissions. Avoid using your car whenever possible. You'll be helping the environment by preventing acid rain [source: National Geographic].
- Alternative fuels An excellent way to prevent acid rain is to stop using nonrenewable fuels and switch over to renewable sources of energy, such as solar, wind and water energy. As the technology for these alternative energies increases, they will become more accessible to the public. Try using solar powered heating systems and battery-powered cars to do your part for the environment [source: EPA].
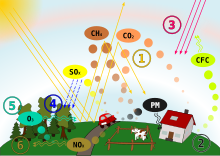
Acid Rain pathway
[copied]



It is very informative topic for us
ReplyDeleteGood information
ReplyDeleteThanks for reading my topic
ReplyDeleteNice article
ReplyDeleteInformative post👍
ReplyDeleteImportant article... Keep it on 👍🏻
ReplyDeleteThanks😊
Delete👍
ReplyDelete